eCO Study mobile application and infrastructure are used by healthcare providers and their adult female patients with recurrent ovarian cancer who are being treated with the investigational drug combination of Olaparib-Cediranib.
eCO Study mobile application is distributed for clinical study purpose only and therefore, is intended for prescription use and will not be indicated for over-the-counter use or be available for download and use without activation by the study staff.
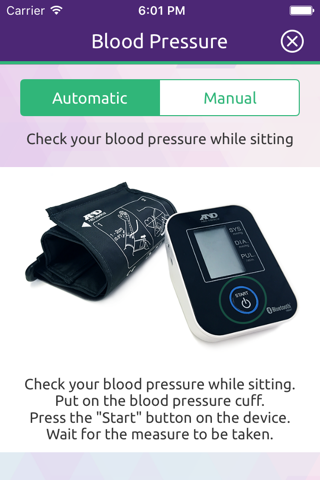
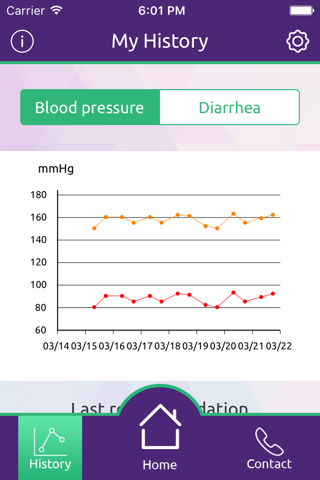
eCO Study mobile application provides secure capture, storage and transmission of blood pressure and diarrhea data as well as other symptom-related data to the study team to aid in remote monitoring of these symptoms by health care professionals.
eCO Study supports symptom monitoring by HCPs through analysis and reporting of the symptom-related data reported by the patient via the app. In addition, it provides directions to patients related to symptom management which are the same as directions that physicians provide to patients as part of routine clinical practice.
eCO Study is not intended to replace the care provided by a licensed healthcare professional, including prescriptions, diagnosis, or treatment.
CAUTION -- Investigational device. Limited by Federal (or United States) law to investigational use.